Long COVID: 17 Aug. 2024 Lancet Update
By The Lancet - Human Synthesis - 22 December 2024
Summary
Post-COVID-19 condition (also known as long COVID) is generally defined as symptoms persisting for 3 months or more after acute COVID-19. Long COVID can affect multiple organ systems and lead to severe and protracted impairment of function as a result of organ damage. The burden of this disease, both on the individual and on health systems and national economies, is high.
In this interdisciplinary Review, with a coauthor with lived experience of severe long COVID, we sought to bring together multiple streams of literature on the epidemiology, pathophysiology (including the hypothesised mechanisms of organ damage), lived experience and clinical manifestations, and clinical investigation and management of long COVID.
Although current approaches to long COVID care are largely symptomatic and supportive, recent advances in clinical phenotyping, deep molecular profiling, and biomarker identification might herald a more mechanism-informed and personally tailored approach to clinical care. We also cover the organisation of services for long COVID, approaches to preventing long COVID, and suggestions for future research.
Introduction
More than 4 years after the COVID-19 pandemic began, millions of people continue to suffer long-term sequelae of SARS-CoV-2 infection.1,2 Yet, despite thousands of academic papers (including 170 systematic reviews) mentioning “long COVID”, “post-acute (sequelae of) COVID-19”, “chronic COVID-19”, or “post-COVID-19 condition” in their titles or abstracts, many clinicians remain unsure of how to evaluate and manage individuals with post-COVID-19 condition (also known as long COVID).
Reasons for this uncertainty include conflicting definitions; the existence of multiple putative pathophysiological mechanisms; the lack of a single, agreed upon and accessible biomarker that could be used for diagnosis, monitoring, and research; and changes in the natural history of this condition over time caused by (for example) viral evolution, vaccination, and novel therapeutics. All of these reasons are reflected in the sheer volume of research already published and the pace at which new papers are appearing.
There are signs that virological, immunological, and other basic science research appears to be close to producing clinically relevant breakthroughs in diagnosis and treatment3–7 to augment current clinical practice, which is based largely on a rehabilitation therapy model of alleviating symptoms and optimising functional performance.Particularly in the early months of the pandemic, many people living with long COVID were undiagnosed, disbelieved, inadequately assessed, or inappropriately treated,8,9 an experience some individuals described as “medical gaslighting”.10
The dearth of clinical knowledge and scarcity of services at that crucial time helps explain the rapid emergence of online communities, which fulfilled important roles in mutual support, information provision, activism, and research.8,11–13In this interdisciplinary Review, we had three goals. First, to make sense of the extensive research literature on long COVID, including literature on epidemiology, basic science, lived experience, and clinical trials of therapy.
Second, to bring this state-of-the-science summary into dialogue with current approaches and dilemmas in clinical practice. And third, to acknowledge and respond to the call “for patients’ ongoing contributions to be recognised and used to combat the suffering of multitudes”.9
Definitions
The persisting sequelae and longer-term complications of COVID-19 were named long COVID by patients on May 20, 2020;9 the term was widely taken up and used by people living with these sequelae.14 The term long COVID, defined somewhat vaguely, was later formally adopted by public health bodies in the USA,15 although WHO uses the term post-COVID condition16 and the UK National Institute for Health and Clinical Excellence17 prefers ongoing symptomatic COVID-19 and post-COVID-19 syndrome. These terms are defined in table 1. None of them requires a positive laboratory or lateral flow test.
| Name | Definition | |
|---|---|---|
| WHO16 | Post-COVID condition | Usually, 3 months from the onset of COVID-19; symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis in individuals with a history of probable or confirmed SARS-CoV-2 infection |
| US Department of Health and Human Services15 | Long COVID | Signs, symptoms, and conditions that continue or develop after initial COVID-19 infection and last more than 4 weeks |
| UK National Institute for Health and Clinical Excellence17 | Ongoing symptomatic COVID-19 | Symptoms that are unexplained by an alternative diagnosis and persist for 4–12 weeks after acute COVID-19 |
| UK National Institute for Health and Clinical Excellence17 | Post-COVID-19 syndrome | Symptoms that are unexplained by an alternative diagnosis and persist for more than 12 weeks after acute COVID-19 |
Table 1Some formal definitions covered by the term long COVID
The absence of consensus on a name or definition partly reflects prevailing confusion about underlying disease processes and natural history. Research would benefit from greater consensus on definitions, and ideally, such definitions should reflect pathological mechanisms. However, different definitions might be appropriate for different non-research purposes (eg, clinical care and monitoring, service planning, peer support, and activism).
Epidemiology and risk factors
Epidemiological studies on long COVID are difficult to interpret and combine because they have widely different sampling frames, inclusion criteria, participant demographics, diagnostic criteria, and methodologies.18 They include, for example, follow-up studies of people discharged from hospital (skewed towards those with more severe initial illness)19–22 or in military veterans’ insurance schemes (skewed towards older men),23
Surveys of people who had joined online support groups (skewed towards individuals with higher levels of education whose symptoms were troubling them sufficiently to seek peer support),24 retrospective25–27 and prospective28 analyses of electronic health records (skewed towards individuals with a coded diagnosis), post-mortem studies (skewed towards people who had died),29,30 case series from specialist clinics (skewed towards people who had been referred),31,32 and community-based surveys (skewed towards people who return questionnaires).33–35
Long COVID in unselected primary care populations is hard to study because many people did not present to their primary care physician at all and those who did were rarely diagnosed or coded as having long COVID.28 The condition is unrecognised or under-recognised in some parts of the world, and there are very few studies of long COVID in low-income or middle-income countries.1,36
Notwithstanding the limitations of some observational cohort studies, there are now several important completed or ongoing longitudinal long COVID cohorts including large and representative community samples (eg, the UK Office of National Statistics [ONS]37,38 and the Real-Time Assessment of Community Transmission study [also known as REACT]33,35 from the UK), hospital follow-up cohorts (eg, the Post-Hospitalisation COVID-19 study [PHOSP-COVID] in the UK,3 Veterans Administration in the USA,23,39 and an early cohort recruited from Wuhan, China19,40,41), and some mixed community and hospital studies (eg, the Researching COVID to Enhance Recovery initiative [also known as RECOVER] in the USA).42,43
Estimates of the incidence of long COVID after acute infection range from 50–85% for unvaccinated people who were hospitalised,23,44 10–35% for unvaccinated people who were not hospitalised,23,37,44 and 8–12% for vaccinated individuals.37,42,45,46 Two recent meta-analyses concluded—controversially—that long COVID occurs in 45%47 and 57%48 of people after acute COVID-19 infection, but such unqualified grand means do not take account of sampling and other biases in primary studies or the attenuating effect of vaccination.33,46,49
These figures should also be interpreted in the context that no definition of long COVID includes a minimum level of symptom severity or functional impairment and current definitions still diverge on symptom duration (4 weeks, 6 weeks, 12 weeks, 3 months, 6 months, or a year since the acute infection). A person with a single mild symptom, which bothers them little, contributes as much to an estimate of incidence or prevalence as someone with multiple severe symptoms who is unable to leave their home.
According to UK ONS survey data, 20% of people reporting long COVID symptoms describe them as “severe”.37Figure 1 shows ONS data for the prevalence of long COVID symptoms 12 weeks or more after acute COVID-19 infection in a large sample of people (adults and children) from England and Scotland collected in February and March, 2024; the prevalence of long COVID in this population was 1·8% (if including people with symptoms between 4 weeks and 12 weeks, the prevalence is 3·3%).38
People aged 35–65 years were most affected. In people who self-classified as “inactive, not looking for work”, the prevalence of long COVID lasting beyond 12 weeks was 5·7%, confirming findings from studies showing that this condition is having a substantial effect on people's ability to work in paid employment.50–53 Of the individuals who self-reported long COVID and provided a date, 71% had had symptoms for at least 1 year, 51% for at least 2 years, and 31% for at least 3 years.38 34% of respondents did not state how long they had had symptoms so the data in figure 1 relate to the 66% of respondents who did.
01136-X/asset/5148d8a6-c5a6-48f7-bc43-6753f3503afd/main.assets/gr1.jpg)
Epidemiological studies have identified that some demographic groups (eg, women aged 35–50 years, socioeconomically deprived individuals), people with particular medical conditions (including type 2 diabetes, allergies, a past history of post-viral fatigue, asthma, chronic lung disease, heart failure, and chronic kidney disease), individuals who had a more severe acute illness, people with high BMI, and unvaccinated individuals are at higher risk of long COVID than others and are more likely to have severe symptoms.12,24,32–34,39,42,54
However, as noted earlier, these comparative statements are based on sparse empirical evidence collected from a wide variety of settings and study designs of variable quality. Hence, the epidemiological evidence should be viewed with caution.Notwithstanding statistically significant differences among demographic groups (figure 1), long COVID can occur in all ages, genders, ethnic and racial groups, in people who were previously healthy and fully vaccinated, and in individuals whose acute illness was mild or even asymptomatic.12,28,33,55–57
Because hospitalisation for acute COVID-19 was always uncommon (2·5% of all cases in 2020 and 1·3% of all cases in 2021)37 and is now rare, most people now living with long COVID had a mild or moderate initial illness that did not result in hospitalisation.23,47,48 One confounding factor in the early months of the pandemic was that many people who would have benefited from hospitalisation did not seek care or were turned away due to poor understanding of the disease or rationing of care.58The association between age and long COVID is inconsistent across studies.59
This inconsistency probably reflects influences such as high mortality in the first waves of the pandemic and subsequent high rates of vaccination in older adults, lifestyle factors, access to services, age-specific comorbidities (eg, cognitive impairment), and atypical presentation along with few age-sensitive questionnaires to detect key symptoms. A few studies comparing variants of SARS-CoV-2 have shown that long COVID was more common following the original alpha (B.1.1.7) and delta (B.1.617.2) strains than the omicron (B.1.1.529) strain.60,61
However, the massive numbers of omicron cases probably resulted in larger absolute numbers of people developing long COVID after infection with omicron. Some studies have found similar patterns and severities of symptoms among the SARS-CoV-2 variants,60,61 whereas others have found differences (eg, less anosmia and more myalgia and mental health symptoms with omicron).62 These data are mediated by the effects of vaccination, immunity from previous infection, and better management of acute COVID-19 (including targeted antivirals for individuals at highest risk). Figure 2 summarises the risk factors for long COVID.
01136-X/asset/cae1f60f-053e-4437-a0ce-753f1bc67eb5/main.assets/gr2.jpg)
Symptoms of long COVID and their impact
Manifestations of long COVID are heterogeneous, multisystemic (the condition can affect any and all organ systems), and can change over time. But patterns that are both diagnostically and prognostically important can usually be discerned through a careful history-taking process (panel 1).
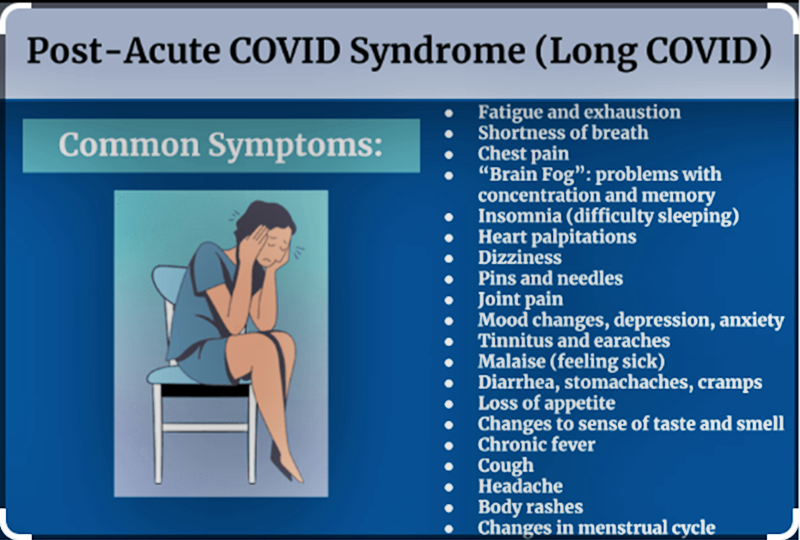
Panel 1Long COVID: a distinctive narrativePeople with long COVID typically recount an initial acute illness that was either paucisymptomatic (ie, with one or a few symptoms—perhaps cough, fever, and breathlessness) or multisymptomatic (ie, with multiple symptoms, which might include shortness of breath, chest pain, cognitive impairment, loss of smell and taste, profound fatigue, muscle and joint pain, gastrointestinal upset, headache, and rashes),32 although long COVID has also been documented after asymptomatic COVID-19.63
Following acute COVID-19 infection, people might describe partial or even complete—but temporary—recovery before developing a set of symptoms, either similar to or different from the original illness, which some individuals describe as “strange”,8 energy-sapping and, in many but not all cases, fluctuating.8,64 The underlying pathological mechanisms driving these symptoms are complex and described in panel 2.A common symptom in long COVID is fatigue,42,65 which classically becomes worse following physical or mental exertion (post-exertional symptom exacerbation).66
Fatigue might be associated with sleep disturbance (especially, unrefreshing sleep and a sensation of being too tired to sleep),67 exacerbations of pain,68 and blunting of cognitive function (ie, brain fog),69 particularly in relation to memory and higher order functions such as multitasking and making complex judgements.70 People who were admitted to hospital (most usually for acute respiratory distress) or seen in an emergency department during their acute COVID-19 illness are more likely to go on to develop persistent respiratory symptoms, especially breathlessness, although these symptoms might occur in any patient.71
There might be a characteristic pattern of respiratory symptoms—a constricting or burning sensation in the chest, a sense of not getting enough air in, not being able to fully breathe out, and feeling “gaspy”.72 Some but not all such cases could fit what clinicians call breathing pattern disorder.72Accompanying allergic symptoms can include rashes (eg, hives), streaming eyes, or blocked nose. Throat and upper gastrointestinal symptoms include altered voice, difficulty swallowing, and nausea, which, combined with persistent inability to smell or taste food, can lead to altered eating patterns, loss or gain in weight, and nutritional deficits; lower gastrointestinal symptoms can include bloating and diarrhoea.73
Dizziness with hypotension on standing (orthostatic hypotension) or tachycardia without hypotension (postural orthostatic tachycardia syndrome) can be traced back to disturbed autonomic function (dysautonomia).74–76 Anxiety and depression often accompany long COVID, especially if multisymptomatic, and teasing out whether the mental health condition or conditions preceded or followed the physical manifestations can be difficult.77,78 Older people might present atypically and non-specifically.79 Sarcopenia can accompany persistent long COVID in people of any age.79–81
A striking characteristic of long COVID is functional impairment: individuals find they cannot do what they could previously do.82 Many cannot work a full 8-hour day, resulting in withdrawal from the workforce if adjustments and phased returns cannot be accommodated. In severe cases, people are unable to undertake activities of daily living such as washing and dressing, or they find these basic activities so draining they require rest afterwards.The course of long COVID varies. Recovery can progress at different rates, and some people experience periods of apparent recovery followed by relapse.
The chance of recovery is highest in people who had a less severe acute illness, are in the first 6 months after that illness, and were vaccinated; people whose illness has lasted between 6 months and 2 years are less likely to fully recover.19,23,40,41,46 There is little published research on people who have had long COVID for 2 years or more, but their chances of full recovery appear low.38 At this stage, the condition typically relapses and remits with compromised quality of life.19,23,40,41 People with persistent long COVID face substantial economic burden from their inability to work, either at their premorbid level or at all.50,51
Long COVID can be caused or complicated by organ damage or systemic stress that occurred in the acute phase or emerges anew in the post-acute phase (eg, pulmonary embolism, stroke, myocardial infarction, acute kidney injury, hepatobiliary injury, Guillain-Barré syndrome, or sepsis).83,84 Compared with people who were not infected, the risk of death or hospitalisation is increased for at least 12–24 months after the acute illness, especially but not exclusively in people who were hospitalised or had severe symptoms during their initial COVID-19 illness.23,40,85
Clinicians should be alert to the increased risk of organ damage, including clot formation and downstream acute infarction consequences, in the subsequent months and even years, especially in the context of multiple SARS-CoV-2 reinfections.23,86 New symptoms that emerge with time might reflect respiratory,87 cardiovascular,55,75 neurological, 29,71,88 musculoskeletal,89 autoimmune,90 and generic (eg, myalgic-encephalomyelitis-like)91 sequelae or the effect of reinfection.40 Some people will also have long-term sequelae of medical trauma (eg, post-intensive care syndrome and post-traumatic stress disorder).92,93
Many but not all people with long COVID have pre-existing conditions (including asthma, allergies, attention deficit hyperactivity disorder, musculoskeletal pain, diabetes, poor mental health, insomnia, headaches, chronic fatigue, and frailty), which can exacerbate—and be exacerbated by—long COVID.32,79,94 When comorbidities are present, management requires a personalised approach that takes both long COVID guidance17,95 and other relevant factors (eg, patient priorities, practicalities, and the need to avoid investigation fatigue and polypharmacy) into account.96
There are many parallels between long COVID and other known or suspected infection-associated chronic syndromes (also known as post-acute infection syndromes), including the sequelae of other coronaviruses (SARS-CoV and MERS-CoV), West Nile virus, Epstein–Barr virus, and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS).97–100 Given these examples, the increased morbidity and mortality from organ damage in COVID-19 will possibly continue for years (in West Nile Virus, for example, all-cause mortality was significantly elevated for at least 8 years after infection).101
Long COVID—one disease or many?
Initial advancements in our understanding of long COVID involved detailed analyses of patient-reported symptoms from surveys and electronic health records. These studies, most of which were undertaken by clinicians and did not include metabolic profiling, generated various long COVID phenotypes on the basis of symptom clusters (see examples in table 2, which are listed by sample size).3,32–34,42,43,102–115 Clinical phenotyping studies applied different methods to different samples and—unsurprisingly—therefore produced different cluster patterns (or no clusters at all).
| Country | Study design and setting | Sample | Symptom clusters | |
|---|---|---|---|---|
| Prieto-Alhambra et al (2024)102 | Multinational | Big data study combining seven different national databases (covering primary care, secondary care, and claims) with latent class analysis | Data on 787 078 individuals from Estonia, France, Netherlands, Norway, Spain, UK, USA | Most people with a long COVID coded diagnosis had only a single symptom; of the individuals with multiple symptoms, three clinically relevant clusters were apparent across all or most of the 7 databases: anxiety and depression (more common in women aged between 20 and 30 years, and accompanied in some databases by fatigue, headache and allergy); abdominal pain, gastrointestinal issues (in both sexes, more common in individuals aged between 20 and 40 years); and cough, dyspnoea, fatigue, joint pain, and chest pain (more common in men aged between 40 and 60 years) |
| Whitaker et al (2022)33 | UK | REACT-2 postal survey | 28 713 participants who experienced some symptoms at 12 weeks after COVID-19 | Two main clusters at 12 weeks: fatigue predominant, with myalgia, difficulty sleeping, and breathlessness; and breathlessness predominant, with chest tightness or chest pain, often with fatigue |
| Thaweethai et al (2023)42 and Reese et al (2023)43 | USA | Prospective observational cohort (RECOVER study) in 33 US states enrolled before April, 2023 (no start date given) | 9764 participants from hospital and community clinics (all with long COVID); 71% female, 29% male, 16% Hispanic or Latino, 15% non-Hispanic Black, and 69% White; median age of 47 years; 10% had been hospitalised for acute COVID-19 | In increasing order of severity: loss of taste and smell, with less fatigue (66%) and post-exertional malaise (55%) relative to clusters 2–4, each of which had a very high prevalence of both (84–94% fatigue and 94–99% post-exertional malaise); chronic cough, gastrointestinal, palpitations, thirst, no brain fog or loss of smell or taste; brain fog without loss of smell or taste; and high prevalence of most symptoms, especially palpitations, hearing issues, gastrointestinal issues, thirst, joint pain, muscle pain, weakness, dizziness, brain fog, headache, and reduced sexual desire or capacity |
| Wong-Chew et al (2022)103 | Mexico | Telephone survey of patients discharged from temporary hospital for acute COVID-19 | 4670 participants; median age of 48 years; 51% female and 49% male; no ethnicity data | 45 symptoms across eight symptom clusters: neurological; mood disorders; systemic (eg, generalised tiredness); respiratory; musculoskeletal; ear, nose, and throat; dermatological; and gastrointestinal |
| Fernández-de-las-Peñas et al (2023)104 | Spain | Follow-up of a random sample of patients hospitalised for COVID-19 in public hospitals between March and May, 2020 | 1969 participants (with 98% response rate); mean age of 61 years; 46% female and 54% male | In increasing order of severity: few and mild symptoms, no functional limitation (also lower pre-COVID-19 co-morbidities); fatigue, breathlessness on exertion, and some functional limitation; and severe fatigue, breathlessness at rest, anxiety and depressive symptoms, sleep disturbance, and severe functional limitations |
| Zhao et al (2023)105 | China | Questionnaire sent to patients who had had laboratory-confirmed COVID-19 between October and November, 2021 | 1000 participants (with 56% response rate); 56% had moderate long COVID symptoms (the categories none and 1 [mild] were combined); 55% female and 45% male; mean age of 56 years; no ethnicity data; 8·5% were severe or critical in the acute phase | In increasing order of severity: no or mild sequelae (median 1 symptom); moderate sequelae with mainly physical impairment (median 4 symptoms); moderate sequelae with mainly cognitive and mental health impairment (median 5 symptoms); and severe physical, cognitive, and mental health impairment (median 11 symptoms) |
| Goldhaber et al (2022)34 | USA | Electronic survey of patients in a health insurance scheme who tested positive for COVID-19 between March, 2020, and July, 2021 | 999 respondents (with 10% response rate); 49% had long COVID symptoms; mean age of 51 years; 56% female and 44% male; 53% White, 9% Asian, 5% Black or African American, 20% mixed race, 10% unknown race; 7% hospitalised | Five clusters: predominantly gastrointestinal; predominantly musculoskeletal (more common in older people and mixed race groups); predominantly neurocognitive (associated with depression and anxiety); predominantly airway; predominantly cardiopulmonary |
| Ito et al (2024)106 | Japan | Multicentre, prospective cohort recruited from hospital clinics from January, 2020, to February, 2021 | 935 participants assessed 3 months after a diagnosis of long COVID | Five clusters: no or minor symptoms; loss of smell or taste; fatigue and dyspnoea; fatigue, so-called psychoneurotic symptoms and dyspnoea; and numerous symptoms across multiple organs |
| Torrell et al (2024)107 | Spain | Online survey undertaken in collaboration with the Catalan long COVID patient support group | 905 participants (80% female and 20% male); most had not been hospitalised with acute COVID-19; none had been in an intensive care unit | Five clusters: loss of taste and smell; heterogeneous (many symptoms across many organ systems, none predominant); multisystemic (several organ systems affected); multisystemic and predominantly dysautonomic; and menstrual and sexual alterations |
| Liew et al (2024)3 | UK | Clinical assessment plus biomarker (proteomic) profiling | 657 adults hospitalised for acute COVID-19 and followed up in the PHOSP-COVID study | Five overlapping clinical clusters with some mapping to inflammatory biomarkers: fatigue; cardiorespiratory symptoms; gastrointestinal symptoms; cognitive impairment; and anxiety or depression |
| Tsuchida et al (2023)108 | Japan | Prospective observational cohort enrolled between January, 2021, and May, 2022 | 497 participants, all referred to a hospital long COVID clinic; 57% female and 43% male; mean age of 42 years; no ethnicity data | In increasing order of severity: fatigue only; fatigue, dyspnoea, chest pain, palpitations, and forgetfulness; and fatigue, headache, insomnia, anxiety, motivation loss, low mood, and forgetfulness; other symptoms were mainly hair loss and taste and smell disorders |
| Van den Houdt et al (2024)109 | Netherlands | Online self-report questionnaire (114 symptoms) analysed with latent class analysis | 458 participants with long COVID recruited via the Prolific platform of people willing to take part in research | Six clusters: moderate inflammatory symptoms; high inflammatory symptoms; moderate malaise–neurocognitive symptoms; high malaise-neurocognitive-psychosocial symptoms; low overall symptoms; and high overall symptoms |
| Sivan et al (2022)110 | UK | Condition-specific screening tool and outcome measure C19-YRS completed by participants in a community rehabilitation service | 370 participants; mean age of 47 years; 64% female and 36% male; median duration of symptoms of 211 days | Three distinct severity types based on symptom score on a 0–10 scale: mild (n=90; mean symptom score <3); moderate (n=186; mean score 3–6); and severe (n=94; mean score >6); cluster analysis did not identify distinct symptom-based phenotypes; a further longitudinal study confirmed that this severity cluster pattern was maintained over time111 |
| Mateu et al (2023)32 | Spain | Prospective, observational cohort enrolled between May, 2020, and February, 2022 | 341 participants with long COVID (from a total cohort of 548 participants), all recruited from a hospital COVID-19 clinic; mean age of 48 years; 70% female and 30% male; 86% White; 38% hospitalised | In increasing order of severity: predominantly fatigue; fatigue plus dyspnoea, neurocognitive complaints, headache, myalgia, arthralgia, chest pain, and tachycardia; and fatigue, dyspnoea, neurocognitive complaints, headache, myalgia, arthralgia, chest pain, and tachycardia plus skin and smell alterations, dysphagia, diarrhoea, and neurosensitive symptoms |
| Fischer et al (2022)112 | Luxembourg | Predi-COVID, a prospective cohort study of people in Luxembourg with a PCR-confirmed diagnosis of COVID-19; recruited from May, 2020, to May, 2021 | 288 participants; mean age of 43 years; 59% female and 41% male; no ethnicity data; 3% hospitalised. | In increasing order of severity: few and mild symptoms (mean 2·9), also fewer comorbidities and less severe initial infection; many and moderate symptoms (mean 11); and many and severe symptoms (mean 18), including vascular, urinary and skin, plus severe functional impairment; more likely in women and smokers |
| Kenny et al (2022)113 | Ireland | Questionnaire as part of the All-Ireland Infectious Diseases prospective cohort study; recruited between March, 2020, and April, 2021 | 233 respondents; 77% with mild initial disease; 74% female and 26% male; 80% White; median age of 43 years; 32% hospitalised | In increasing order of severity: few and mild symptoms; predominantly pain, especially joint pain, myalgia, and headache; and preponderance of cardiovascular and respiratory symptoms (chest pain, shortness of breath, and palpitations) |
| Kitsios et al (2024)114 | USA | Structured telephone interview | 214 participants studied approximately 200 days after a long COVID diagnosis | Three clusters: high-burden constitutional symptoms; loss of smell or taste; and minimal residual symptoms |
| Woodruff et al (2023)115 | USA | Clinical assessment plus deep molecular profiling with Olink proteomics | 97 participants with long COVID; 26 control participants (with uncomplicated recoveries after acute COVID-19) | Two distinct clinical clusters that mapped to biomarker profiles: inflammatory post-acute COVID-19 syndrome (fatigue, dyspnoea, muscle weakness, and progressive myalgia); and non-inflammatory post-acute COVID-19 syndrome (fatigue, dyspnoea, and brain fog) |
Table 2Symptom clusters in long COVID derived from cohort studiesPHOSP-COVID=Post-Hospitalisation COVID-19. REACT=Real-Time Assessment of Community Transmission. RECOVER=Researching COVID to Enhance Recovery.
In all studies, there was considerable overlap between clusters, which is consistent with (but does not prove) the hypothesis that long COVID is, broadly speaking, “a single, multisystemic multifaceted post-viral disease rather than different pathologically-independent subsyndromes”.32 However, an alternative hypothesis is that multiple discrete pathological processes do exist but produce overlapping phenotypes (eg, fatigue might have more than one pathological pathway), as we discuss in panel 2.
Panel 2Pathological mechanisms in long COVIDAn extensive research literature from basic science reveals various biochemical and cellular pathways that are disrupted in people with persistent and severe long COVID symptoms.4,116,117 Three main clusters of primary mechanisms are strongly supported by current evidence.
The first comprises virus-related mechanisms that include persistence of the SARS-CoV-2 (replication-competent) virus or, more probably, its components (proteins S and N) in tissues,118–121 which would directly damage target tissues and organs, and reactivation of other viruses such as Epstein-Barr and other herpesviruses;97,122,123 this persistence could be caused by ineffective immunity124 or other mechanisms.
The second comprises immunoinflammatory mechanisms that include dysregulated immune response (eg, exhausted T-helper cells, elevated cytotoxic T cells, elevated cytokines, and the appearance of aberrant immune cell subsets)12,123,125 and consequent immunopathology, which destroys or injures bystander tissues or autoimmunity122 with its consequences.
The third includes endothelial inflammation and immune thrombosis.126Both immunoinflammatory mechanisms and endothelial inflammation and immune thrombosis can cause or exacerbate dysregulation of the complement cascade and complement-mediated tissue injury.127 Other mechanisms that have been invoked to explain long COVID pathogenesis include protein misfolding caused by viral proteins such as N and S128,129 and altered oral and gut microbiomes.125,130–132
Technically, however, these two mechanisms are more likely to be secondary manifestations of one of the three primary mechanistic clusters (misfolding as a consequence of virus-related mechanisms; altered microbiomes due to virus-related or immunoinflammatory mechanisms or both).
The same is probable for other proposed mechanisms, including down-regulation of mitochondrial genes essential for energy metabolism133 (common in cytokine dysregulation disorders and in part responsible for fatigue), dysregulated circadian rhythms,134 autonomic dysregulation,135 deranged endocrine functions,123 dysfunctional neurological signalling (especially in the brainstem and vagus nerve),88 an imbalance of serotonin in the brain,136 vascular disruption in the blood–brain barrier,137 abnormalities in skeletal muscle structure, exercise-induced myopathy, and tissue infiltration of amyloid-containing deposits.138
Genetic susceptibility59 almost certainly plays some role, and there is a need for large-scale genomic sequencing studies to identify not only the genetic risk and protection factors, but also the underlying pathogenetic mechanisms. Immune abnormalities differ by sex (as does the propensity towards autoimmunity) and might explain some of the sex differences in long COVID symptomatology.139Importantly, the above mechanisms might coexist and feed into one another. A persistent reservoir of viral proteins or RNA, for example, could stimulate both innate and adaptive immunity, causing immunopathogenesis.
Via tissue damage, it could further fuel autoimmunity in susceptible individuals, and inflammation could affect endothelial dysfunction, thrombosis, and altered metabolism. Viral molecules could further directly damage cell and tissue function, or cause protein (Panel 2 continues on next page) (Continued from previous page)misaggregation. There are no definitive data on the role of clotting abnormalities in long COVID and scholars are divided on this issue;140,141 ongoing research studies142 might help resolve these debates.
A key question driving development of therapeutic interventions is whether and to what extent and duration the virus or its components remain present (and hence can be targeted with vaccines, antivirals, or neutralising monoclonal antibodies). If not, therapies must focus on the aftermath of virus interactions with the host (eg, anti-inflammatories for tissue inflammation; immunotherapy for immune subset changes; immunosuppresion for autoimmunity; anticoagulation or apheresis for thrombosis; faecal transplant, dietary therapy, or probiotics for gut dysbiosis; and Epstein–Barr vaccine for reactivation).4
In the future, findings from mechanism-focused studies might enable a so-called treatable traits approach to personalising management of long COVID, with therapies directed against particular symptom clusters and monitored with appropriate biomarkers.143,144Clues on pathogenetic mechanisms are likely to emerge from well performed and incisive deep molecular studies, which have the potential to inform pragmatic, adaptive clinical trials. Such studies are beginning to appear.
Liew and colleagues,3 for example, reported on a sample of patients from the UK Post-Hospitalisation COVID-19 (also known as the PHOSP-COVID) study (all of whom had been hospitalised with acute COVID-19); they delineated five distinctive metabolomic profiles in people with long COVID and fatigue, cardiorespiratory symptoms, gastrointestinal symptoms, cognitive impairment, and mental health symptoms.3 In a much smaller but very detailed study, Woodruff and colleagues115 studied 97 individuals with long COVID and a comparator group of 26 individuals with uncomplicated recovery.
With the help of Olink proteomics and machine learning, they found that two main phenotypes segregated sharply on the basis of only 12 biomarkers: inflammatory post-acute sequelae of COVID-19 (with various atypical biomarkers, a clinical picture of fatigue with myalgia, and slow or no recovery) and non-inflammatory post-acute sequelae of COVID-19 (with biomarker profiles similar to control individuals, fatigue without myalgia, and a greater likelihood of recovery).115
Along similar lines, Cervia-Hasler and colleagues127 have recently identified dysregulation of complement metabolism in the acute phase of COVID-19 as a strong predictor of long COVID at 6 months, and Krishna and colleagues145 found that persistent interferon-γ release predicts long COVID symptoms. Its decrease to concentrations in the standard range is associated with symptom resolution.145Some therapies for long COVID that are based on putative biological mechanisms of disease are listed in the appendix (p 1).
Many front-line clinicians view such therapies as having little evidence of efficacy; they await the results of randomised controlled trials, of which there are over 400 ongoing at the time of writing;146 major trials have been summarised elsewhere.146–148 Some ongoing or proposed clinical trials have been challenged on the grounds that the interventions being tested do not reflect mechanistic evidence about the underlying pathology of long COVID.149
Principles of clinical management
A full clinical history should be taken when a patient first presents. Given the absence to date of a definitive and available biomarker, the patient's own detailed chronological account is crucial to making the diagnosis, affirming the illness, and establishing the trust on which an ongoing therapeutic relationship depends.
The history should document the patient's pre-morbid health status; the number of infections; the severity and duration of the acute COVID-19 episode or episodes; the patient's vaccination status; how their symptoms have evolved and fluctuated; and how symptoms have affected their work, home life, and social relationships. A condition-specific patient-reported outcome measure (PROM), such as C19-YRS,150 is essential, as people might not report all symptoms unless specifically asked. Many long COVID symptoms are non-specific or overlap with those of conditions that commonly coexist (or might be confused) with long COVID, such as chronic musculoskeletal or rheumatological conditions, chronic respiratory conditions, type 2 diabetes, or thyroid disorders.
Long COVID is frequently misdiagnosed (eg, as menopause, common migraine, depression, anxiety, or deconditioning). Although test-confirmed COVID-19 is not part of the definition of long COVID, typical symptoms following a positive test increase confidence in the diagnosis.A careful history and follow-up visits can help document what is often a long and complex trajectory, including investigations and referrals and their outcomes, and also provides patients with the opportunity to make sense of a perplexing array of symptoms across multiple body systems.
Simply learning that one's symptoms can all be explained by long COVID, that other people are similarly affected, that the syndrome has also been observed in other viral infections, that the trajectory of recovery is within expected limits, and that alternative diagnoses have been excluded can be greatly reassuring. Validation of the condition builds a positive relationship between the physician and patient, particularly when there is still considerable disbelief and speculation surrounding it.Clinical investigation and management depend on the duration, nature, severity, and trajectory of symptoms.
In people in the first 6 months after acute infection and with more than minimal symptoms, there is some evidence from a systematic review of research studies151 to support a multidisciplinary approach to rehabilitation, although many primary studies were small and uncontrolled.
Rehabilitation would normally include pacing strategies (avoiding post-exertional crashes),89,152 physiotherapy (and especially breathing exercises), psychological support, cognitive and speech rehabilitation, attention to lifeworld context (such as reasonable adjustments and a phased return to the workplace), olfactory training for anosmia, and dietary advice. Table 3 lists some recommended approaches for common symptoms.80,81,111,153–168 Progress towards anticipated recovery should be monitored with generic and condition-specific PROMs (these are summarised in the appendix p 2).
| Description and impact | Investigations (in addition to full clinical examination) | Management | |
|---|---|---|---|
| Fatigue, low exercise tolerance, deconditioning (eg, post-intensive care unit), and post-exertional symptom exacerbation or post-exertional malaise | A so-called flat battery feeling; unable to do usual activities; trying to do more might worsen symptoms; in some cases, fatigue does not improve with rest; a crash, relapse, or worsening of symptoms (physical, cognitive, or emotional) following physical or mental exertion | Take careful history to document nature of fatigue and timing and triggers of exacerbations; perform blood tests as appropriate to exclude anaemia, renal or thyroid disease, vitamin deficiency (eg, vitamins D and B12), acute phase reactants (eg, C-reactive protein); exclude other causes of fatigue, including sleep disorders and neurological conditions; monitor symptom severity and frequency and pattern of relapses with a standardised instrument (appendix p 3); keep a patient activity diary to record triggers to avoid in future; and consider tests for autonomic dysfunction | Holistic, interdisciplinary management (eg, with a physician, occupational therapist, and psychologist); pacing rehabilitation programme;153,154 support for self-management, including the so-called 3 Ps: prioritising, planning, and pacing;155 and advice on phased return to work and reasonable adjustments |
| Exertional breathlessness | Short of breath predominantly with physical activity | Guided by specific symptoms; monitor with a standardised instrument; perform haemoglobin, spirometry, and lung function tests as indicated, and natriuretic peptides and echocardiogram if heart failure is suspected; perform pulse oximetry and sit-to-stand test for exertional hypoxia;156 perform a chest x-ray if persistent lung damage is suspected or to exclude other causes;157 and perform a D-dimer test if acute pulmonary embolism is suspected (negative result does not exclude chronic pulmonary emboli) | Refer to a relevant specialist if clinically concerned (eg, due to worsening symptoms, resting or exertional hypoxia, unexplained atypical spirometry, or atypical chest x-ray) |
| Altered breathing or breathing pattern disorder | Pressure in chest (referred to as the COVID squeeze), shallow breathing, breathlessness, sense of needing to work harder to take a breath, and air hunger | Exclude other causes of exertional breathlessness (see previous row), especially those of episodic breathlessness (eg, asthma, recurrent pulmonary embolism) | Recommend breathing control exercises, signpost to online or printed resources for breathing pattern disorder (eg, by WHO155); and refer to a specialist if no improvement |
| Chest pain | Pain in specific positions, pain on exertion, chest pain (referred to as lung burn) and pressure (“like an elephant sitting on my chest” according to a patient adviser) | Guided by specific symptoms; for example, consider microvascular angina, myocardial infarction, pulmonary embolism, myocarditis or pericarditis, and costochondritis; perform, for example, echocardiograms, troponin, D-dimer, and oximetry tests, including sit-to-stand test, stress test, and cardiac MRI as indicated | If angina-like features, refer to a rapid access chest pain clinic; if inflammatory pattern, consider colchicine or anti-inflammatory analgesics once other causes have been excluded; if myocarditis is suspected, specialist referral is recommended158 |
| Throat and voice symptoms | Sore or dry throat with choking sensation (referred to as the COVID strangle), and altered voice | Take full history and assess to explore differential diagnosis (eg, COVID-19-related vocal cord pathology, gastro-oesophageal reflux, sinus disease, strained voice, and dehydration) | If not improving, refer to an ear, nose, and throat or speech and language therapist as appropriate (check local protocols) |
| Orthostatic intolerance | Palpitations or dizziness on upright posture due to COVID-induced autonomic dysfunction; autonomic dysfunction might also cause gastrointestinal disturbance and generalised pain | Perform NASA 10-minute Lean Test to check for postural orthostatic tachycardia syndrome159 (some symptomatic individuals with long COVID might approach but not reach the formal threshold criteria for postural orthostatic tachycardia syndrome); conduct investigations as appropriate to exclude differential diagnoses (eg, 24-hour echocardigram and blood pressure tests) | Fluids, electrolytes, compression garments, lifestyle adaptation, and specialist rehabilitation if tolerated; specialist referral for the tilt table test160 if indicated; various drugs are under investigation161 |
| Neurocognitive dysfunction | Brain fog (ie, poor short-term memory, concentration, problem-solving, and executive function) and mental fatigue | Conduct brief cognitive screening test (eg, Mini Mental State Examination);162 conduct fatigue investigations as above; if memory loss pre-dated COVID-19 and is now worsening, follow usual investigations and pathway;163 consider imaging (eg, MRI), electroencephalogram, and expanded cognitive testing | Strategies of pacing and energy conservation, to-do list diary, avoiding multitasking; formal neuropsychological testing is recommended for new cognitive impairment, especially if unable to work or in a safety-critical occupation |
| Dizziness and vertigo | Unpleasant episodes, sensation of the room spinning, and nausea | Take full history to identify timing, triggers and whether the symptoms are resolving; perform clinical examination (eg, for nystagmus, other neurological signs, and postural drop in blood pressure) | Precautionary measures to avoid falls and head tilt and balance exercises; refer to audiology if indicated |
| Loss of smell | Loss of enjoyment of food and mealtimes, and phantosmia (a persistent, disagreeable background smell) | Perform clinical examination to exclude nasal polyps, chronic sinusitis, and rare inflammatory or neoplastic conditions of nasal cavity and cranial nerves | Structured olfactory training (more effective if commenced early);164 experiment with foods and menus; steroid nasal spray might be useful165 |
| Allergy-type symptoms | Skin rashes (eg, urticaria), conjunctivitis, abdominal bloating, and regurgitation | Confirm urticaria clinically (eg, dermographism); if present, might indicate mast cell overactivity; resurgent atopy (eg, hay fever recurring after many years) is common post COVID-19 | Symptom diary to identify triggers (eg, food, medication, or environmental exposure); antihistamines (obtainable over the counter) might help; allergy clinic referral if fulfils local criteria (eg, for anaphylaxis) |
| Poor sleep | Unrefreshing sleep, exhaustion, exacerbation of fatigue and brain fog, and vivid dreams or nightmares | Assess daytime somnolence (eg, with Epworth sleepiness scale);166 exclude underlying causes (eg, obstructive sleep apnoea with STOP-Bang questionnaire);167 consider sleep study; and assess psychological health; COVID-19-related sleep disorder often overlaps with autonomic dysfunction and mast cell disorder | Sleep hygiene (eg, structured routines, exercise as able, avoiding shift work if possible, and avoiding caffeine and alcohol) and short daytime naps; melatonin might help (exclude other causes before prescribing);168 treatment of sleep apnoea (eg, via positive airways pressure) if indicated by sleep studies |
| Mental health | Anxiety, depression, post-traumatic stress disorder, and loss of identity and purpose | Take full history (hear the patient's story and witness their experience); affirm the reality of their lived experience; carefully distinguish anxiety from postural orthostatic tachycardia syndrome (see above); and assess risk of self-harm and risk to any dependents | Whole-person care; talking therapy, meditation, and medication if indicated, and mental health referral or social prescribing if appropriate |
| Joint and muscle pain | Generalised, focal, or regional pain; might have a coathanger distribution; and might progress to chronic pain | Investigations guided by history and clinical examination; perform C-reactive protein blood tests if inflammatory disorder is suspected, creatine kinase blood tests if myositis is suspected, and additional tests as indicated for rheumatological disorders | Mobilise within personal limits; non-steroidal anti- inflammatory drugs; and neuropathic agents (eg, amitriptyline and gabapentin) in chronic cases, especially if neuropathic symptoms |
| Sarcopenia and osteosarcopenia | Defined as progressive weakness and muscle wasting, with or without bone loss; occurs mainly but not exclusively in older people, leading to disability, functional impairment, cognitive blunting, falls, and reduced quality of life | Perform full geriatric assessment, mindful of potential comorbidities; conduct a falls screen; take a dietary and exercise or activity history; perform social assessment; consider specific tests such as symptom checklists (eg, SARC-F), grip strength and imaging (eg, DXA scan for bone density),80,81 and blood tests as appropriate (eg, haemoglobin, calcium, and vitamin D) | Interdisciplinary rehabilitation to improve respiratory, functional, and cognitive status; dietary support (adequate calories and protein, and supplements if clinically indicated) and exercise (aerobic and endurance) within limits of post-exertional malaise to preserve skeletal muscle mass |
Table 3Management of specific symptoms of long COVIDNASA=National Aeronautics and Space Administration. SARC-F=strength, assistance walking, rise from a chair, climb stairs, and falls. STOP-Bang=snoring, tiredness, observed apnoea, blood pressure, BMI, age, neck size, and gender.
Although exercise has traditionally been a core element of rehabilitation, there is new evidence that unmoderated exercise in long COVID can exacerbate inflammatory and other pathological processes, leading to a worsening of symptoms and delayed recovery.89,152 Pacing-based rehabilitation is therefore likely to be safer and more effective than a standardised and progressive exercise intervention that takes no account of the patient's symptoms on a given day. Exercise specialists working with people with long COVID have made strong arguments in favour of symptom-guided pacing.153
A small, uncontrolled study showed that a structured pacing protocol reduced the frequency of post-exertional symptom exacerbation and increased activity levels and quality of life.154In people whose symptoms have persisted for more than 6 months, considering additional underlying causes and complications and offering symptomatic treatments are important. Patients place high priority on an ongoing therapeutic relationship with a single clinician who can oversee progress and make referrals to different specialties as needed.64 Figure 3 summarises the pathology, presentation, and management of long COVID.
01136-X/asset/5e71e603-a733-47db-b563-7b691111b808/main.assets/gr3.jpg)
Services for long COVID
Services for long COVID vary widely within and between health-care systems. Moreover, they are generally described as insufficient by most people with long COVID, even in countries where health care is free at the point of delivery. In the USA, long COVID is associated with a substantial increase in health-care use and costs.169 In some countries, especially those with poor health-care provision generally, there might be no specific services for long COVID at all (and little public awareness of the condition), and health professionals might be unaware of the condition and not have the training to look for and manage it.1,36
In the UK, there is a tiered approach to service provision, with the most mildly affected individuals encouraged to self-manage (tier 1);155 many of these people will need medical supervision and some might value peer support in online or in-person groups with others with lived experience.170 Tier 2 is care from a generalist physician or general practitioner, who might (if appropriately trained and resourced) manage uncomplicated subacute COVID in the community.
Generalist care includes: hearing and affirming the patient's story; excluding red flag conditions or symptoms; educating and explaining; arranging multidisciplinary team input (eg, community physiotherapy); managing comorbidities; organising investigations and referrals as indicated; working with the patient to set goals and monitor progress against them, including, where appropriate, self-monitoring of symptoms; recommending or prescribing symptomatic remedies; and providing sickness certification.82 Some specific interventions are listed in table 1.
Generalist primary care physicians also have an important role in supporting and advocating for clinically vulnerable people.171 Even when specialist referral is needed for an aspect of long COVID, continuity of care with a single clinician who understands the whole picture and what is at stake for the patient is a crucial element of the overall care package and is greatly valued by patients.64 Those unable to work or care for themselves or their dependents might need social and financial support of various kinds,171 although there is little evidence that such support is currently adequate, especially in low-income countries.172
Referral to specialist services for long COVID (tier 3), if available, might benefit people with more severe symptoms, slower than expected recovery, or specific syndromes (eg, breathing pattern disorder or orthostatic intolerance; table 3). Referral pathways from primary care into such services, and those from a long COVID service to associated specialties (eg, cardiology, respiratory, neurology, rehabilitation medicine, ear nose and throat, psychiatry, or speech and language therapy) should be well defined with clear criteria and protocols.
People with complex sequelae (such as profound and disabling fatigue or complex comorbidities affecting management) might benefit from having their care discussed among multidisciplinary teams of medical and allied professionals. Highly specialised services (tier 4 in the UK) might be needed for people with, for example, cardiovascular complications, autoimmune sequelae, or severe autonomic dysfunction.
Comparative efficacy studies of the tiered model against alternative models (such as specialist care for all patients) are scarce, although in the USA, the Patient-Centered Outcomes Research Institute is beginning to support comparative studies of different models.Although services for long COVID at every level in health-care systems would be ideal, many people even in high-income countries, and particularly people from low-income and racially minoritised backgrounds, are currently unable to access such services.59,171,173–176
People with long COVID in low-income and middle-income countries have been described as receiving little attention and “fragmented care if any care at all” as a result of precarious health systems, under-resourcing of primary care, and the competing double burden of non-communicable and other infectious diseases.36 Overall, few if any countries have fully integrated and comprehensive approaches to long COVID, and in most, the mismatch between need and provision is stark.
Engagement of medical societies to create consensus guidelines for management of long COVID, formalised education programmes for health-care providers, especially those on the front lines of primary care, and partnership with patient groups and patient advocates will be crucial to optimally serve and help the millions of people affected. Given the complex nature of long COVID and the high prevalence of multimorbidity, integration of services is crucial to providing a seamless patient experience.177
Preventing long COVID
Long COVID currently has no definitive cure, so prevention is of the utmost importance. The best way to prevent long COVID is to prevent COVID-19 through well established public health measures such as paying attention to indoor air quality (eg, ventilation or filtration); wearing well fitting, high-filtration masks or respirators when appropriate; and supporting infectious individuals to quarantine.
People with acute COVID-19 should ensure they rest.Vaccination is also crucial. A meta-analysis of primary studies involving 620 221 participants estimated that two doses of vaccine reduces the risk of long COVID by 36·9% and three doses reduces the risk by 68·7%.46 In people who already have long COVID, vaccination has a variable effect on the trajectory of the condition but, overall, reduces the effect of recurrent infections and is therefore recommended in people without contraindications.54,178,179 The updated protein-only vaccines have a theoretical advantage.
Since the severity of the acute illness is a risk factor for developing long COVID, interventions (such as antivirals), which attenuate acute symptoms, should provide at least partial protection against long COVID. This hypothesis is supported by several observational studies. A large US Veterans Administration cohort study showed a statistically significant reduction in risk of long COVID in people given molnupiravir180 or nirmatrelvir181 within 5 days of symptom onset in acute COVID-19.
Another large US study, on Medicare enrolees older than 65 years, found similar results with both these drugs and with nirmatrelvir–ritonavir.182 A retrospective study from Hong Kong suggested a significant protective effect of combined nirmatrelvir and ritonavir, including a statistically significant reduction in post-acute mortality, although the study design is open to confounding.183 Controlled trials of these treatments are currently scarce, however, and none of these drugs has yet been shown to be effective in treating (as opposed to preventing) long COVID.
Molnupiravir appears to be mutagenic and might assist the virus to escape further.184 A pilot randomised controlled trial from Japan of ensitrelvir in acute COVID-19 suggests protection against development of long COVID;185 the results of larger trials are awaited.Given that reinfection is emerging as a substantial contributor to persistent long COVID, ensuring that health-care settings, especially long COVID clinics, are COVID-safe (eg, enforcing mask requirements among clinic staff, air quality measures, and testing protocols) is important.
Conclusions and further research
The evidence outlined in this Review suggests several priority areas for further research.
Vaccines
The hypothesis that protein-based, third-generation vaccines (updated periodically with current strains) might provide the best and most harmless protection from reinfection (and potential long COVID exacerbation) in people with long COVID should be prospectively tested, perhaps in randomised controlled trials. There is a plausible mechanism because such vaccines would avoid presenting the RNA backbone of the virus to the host's immune system, which (in some people) might react exuberantly to its molecular patterns, potentially exacerbating or precipitating long COVID.
As yet, however, there is no empirical evidence of their superiority over other vaccines. The potential association of vaccines, particularly of mRNA or adenovirus vaccines, with clinical presentations similar to long COVID, is addressed in panel 3, and should also be further studied.Panel 3Vaccines and long COVIDAn association between vaccines (including but not only for COVID-19) and neurological sequelae is well documented.186
Antiviral vaccines with key similarities to SARS-CoV-2 (eg, mRNA vaccines) or those that are engineered inside another virus (eg, adenoviruses, as used in the Johnson & Johnson, AstraZeneca, and Sputnik vaccines) could theoretically induce a condition mimicking long COVID in a susceptible host by stimulating an overly exuberant immune response.
The evidence for whether this process actually occurs is scarce. From a focused literature search, we identified 704 results, of which 91 (12%) described some type of vaccine-associated complications. More than 70% of these results were single case reports, suggesting rare occurrence of vaccine-associated complications. As is the case with all vaccines (and drugs in general), side-effects always exist and affect a small number of treated people, but the benefits of vaccination greatly outweigh the risks of the disease in the vast majority of vaccinees.
One issue with whole-virus-based vaccines is the development of thromboembolic complications in a small number of vaccinated people (eg, 1–5 per million administered vaccines with the Johson & Johnson vaccine), which eventually led to the phasing out of these vaccines. A very rare but serious thrombotic thrombocytopenia has also been described.187–189 The other notable adverse effect, also rare but more common with mRNA vaccines, is myocarditis. Incidence of COVID-induced myocarditis following SARS-COV-2 infection was 150 cases per 100 000 or 1000–4000 cases per 100 000, depending on the source; mortality rates in affected individuals varied from 20–70%.190,191
By contrast, the incidence of myocarditis from vaccines (ie, vaccine-related myocarditis) was in the range 0·3–5 cases per 100 000 vaccinated individuals (which is between 30-fold and 10 000-fold less frequent than the incidence from COVID-19 infection), with more than 99% survival. Vaccine-related myocarditis is more frequent in younger male individuals. Mechanistic underpinnings of vaccine-associated myocarditis imply immunopathology or autoimmunity, whereas COVID-induced myocarditis appears to be due to viral cytopathology.
Long-term consequences of this rare complication of COVID-19 vaccines are not well understood; some aspects, including chest pain and dyspnea,190 mimic long COVID and can persist for 6 months or longer.192 A (rare) postural orthostatic tachycardia syndrome-like syndrome has been described following COVID-19 vaccination.193
One small study from Saudi Arabia and Jordan based on self-reports from 498 vaccinated health-care practitioners194 reported a 16% incidence of long-term adverse effects (lasting 6 months or more post vaccination), including fatigue, menstrual disturbances, myalgia, arthralgia, dizziness, and headache; these were more strongly connected to the Sinopharm inactivated vaccine, followed by AstraZeneca and the mRNA vaccines.
A study from Iran, also based on self-reports from 509 vaccinated doctors,195 found the incidence of long-term adverse effects to be 2% in single-vaccine regimens and 6·2% (half of which were cutaneous reactions) in individuals given two combined vaccines; the most common non-cutaneous long-term adverse effects were fatigue and arthralgia.
The authors concluded that the “benefits of COVID-19 vaccination far exceed the potential risks and late [adverse effects] seem to be uncommon”.195 These studies also have limitations that compromise the strength of their conclusions. They had no control groups, so what proportion of participants would have had symptoms without a vaccine is unknown. Some of those participants could have been infected, which would further confound the results. A meta-analysis of 16 primary studies found evidence of menstrual disorders in published papers but also evidence suggesting publication bias.196
Final comment
Overall, rare sequelae of COVID-19 vaccination can overlap with the clinical manifestations of long COVID, but causality has not yet been established. The risk of the former appears orders of magnitude lower than the latter, although the volume and strength of current evidence relating to these risks is weak. Analysis of global adverse event reporting databases for vaccines, along with dedicated clinical and biomedical research, is necessary to further define these effects.
Genetic and epigenetic studies
Genetic susceptibility to acute and long-term sequelae of COVID-19 has been hypothesised on the basis of human leukocyte antigen haplotypes,59,197 which would probably be connected to a propensity to autoimmunity and immunopathology. Epigenetics is the study of which genes are expressed and why; preliminary studies suggest that changes in gene expression (indicated by DNA methylation) persist at least 1 year after acute COVID-19 infection.198 The use of artificial intelligence (AI, ie, deep learning) to elucidate these changes and their implications has begun, but definitive findings are sparse.199,200
Developing predictive biomarkers
Further work on biomarkers, including attention to appropriate control groups, is needed to better target treatments during acute COVID-19 illness and the first 6 months of long COVID. Recently published studies describing distinct clinical patterns and trajectories of long COVID along with distinct molecular profiles115,201 require urgent replication with similar study designs (ie, drawing on machine learning, deep molecular profiling, and incisive clinical phenotyping). Such studies could inform more accurate and consistent definitions and outcome measures.202
Next-generation clinical trials
Future trials should incorporate evidence of biological mechanisms from drug discovery research.7,203 We believe such trials should include (but not be limited to) small, adaptive, and pragmatic designs (typically involving 100–200 participants) to rapidly test many therapies aimed at one or more of the suspected pathogenic mechanisms. These trials should be coupled with AI-supported deep molecular profiling to conclusively discern why a therapy is effective or ineffective. This model, applied iteratively, would be able to narrow down successful candidates to test in large trials.
Long COVID at extremes of age
There is little empirical data on the course and management of long COVID in both children (in whom it is uncommon but can lead to substantial disability and functional impairment)204 and older people (in whom it might be underappreciated and underdiagnosed).79,205
Optimising rehabilitation protocols
There are many unanswered questions relating to current rehabilitation approaches for long COVID. Although, to our knowledge, there are no adequately powered clinical trials comparing graded exercise with symptom-guided pacing, we believe such a trial would now be unethical based on extensive mechanistic evidence89 and an absence of clinical equipoise.153,154
However, pacing as a complex intervention is currently inadequately characterised, so research to refine and test safe adaptive pacing protocols in different cohorts should be a priority. Observational studies could also address the question of whether pacing to avoid post-exertional symptom exacerbation merely stabilises the condition or has the potential to improve it, particularly in individuals with persistent long COVID.
Optimising health services
Given the multisystem nature of long COVID, multidisciplinary models of care based on integrated pathways are often recommended. The efficacy and cost-effectiveness of these models when compared with single-discipline services should be evaluated in different health-care systems. The relative roles of the clinical generalist (eg, primary care physicians and allied professionals) and the long COVID specialist (often but not always a respiratory or rehabilitation physician) in the care of long COVID should be explored and defined.
Education and training for both specialists and generalists should be evidence-based, and guidance and guidelines in this rapidly developing field should be disseminated and updated in a timely way. Recognising the unequal effects of long COVID linked to race, socioeconomic status, and other markers of vulnerability,59,171,173–176 health services research should also explore how to make services optimally accessible and appropriate for disadvantaged and underserved groups.
Search strategy and selection criteria
To identify a manageable sample of studies and reviews, we began with sources known to the authors, focusing particularly on seminal papers (ie, widely acknowledged authoritative sources that were highly cited for their age). We asked leading scholars in the field to recommend additional sources in niche areas, on the basis that such recommendations are a particularly efficient way of identifying key sources.
We also undertook a focused key word search of the US National Library of Medicine's National Center for Biotechnology Information database on April 25, 2024, to identify papers on COVID-19 vaccine-induced long COVID with the keywords “vaccine” AND “induced” AND “long COVID”. We forward-tracked key papers in Google Scholar to identify more recent studies up to April, 2024. To summarise and synthesise these sources, we used a hermeneutic approach—that is, we prepared an initial draft summary and then systematically refined it as we worked through our dataset of papers and discussed how to interpret them.
Further studies of the natural history of long COVID
Ongoing or new longitudinal studies should examine the effect of reinfections and vaccination status on the severity and course of long COVID (including teasing out the differential effects of different vaccines in routinely collected datasets). Another hypothesis that could be further explored longitudinally is whether inflammatory markers in the acute phase of COVID-19 predict longer-term sequelae (and if so, whether targeted use of anti-inflammatory medications or metabolic attenuators [eg, metformin] in the acute phase might attenuate such sequelae in susceptible individuals).
Comparison with other persistent viral syndromes
Basic science research should continue to explore and compare post-acute infection syndromes such as the long-term sequelae of SARS-CoV-2, SARS-CoV, MERS-CoV, and non-coronavirus infections.97 Drawing parallels with, and distinctions from, other conditions will help validate all these syndromes better, thereby consolidating our learning and helping prepare for future outbreaks.
In conclusion, although there is extensive evidence to support multiple interacting biological mechanisms in the pathogenesis of long COVID, most current clinical management is not derived from these biological mechanisms. We believe there is potential for targeted research to close this gap and combat what has been described as the “mass disabling event” of long COVID.206
Contributors
TG and MS undertook an initial search of the literature and produced an early outline of the clinical sections of this Review. They invited JŽN to bring in expertise in basic science and extend the clinical sections. JŽN invited AP (a clinical academic with lived experience of long COVID) to join the author team. All authors contributed to further discussion and extensive iterations of this Review. TG produced initial drafts of the tables, visuals, and appendix, which were amended and extended by all other authors. TG is the guarantor for this Review.
Declaration of interests
In the past 36 months, TG has held research grants from UK National Institute for Health and Care Research, Balvi, Medical Research Council, Health Data Research UK, and Research Council of Norway. She is a Governing Body Fellow at Green Templeton College and a Visitor at the Pitt Rivers Museum, University of Oxford, and was until 2022, a trustee of the Hilda Martindale Charitable Trust (an educational hardship fund).
MS has held research grants from National Institute for Health and Care Research, Research England Policy Support Fund, and the Engineering and Physical Sciences Research Council. He is the Editor-in-Chief of the Oxford Handbook of Rehabilitation Medicine. AP has received consulting fees and grants from the US National Institutes of Health and is Chief Medical Officer of Blooming Magnolia (a 501[c]3 non-profit organisation).
JŽN acknowledges institutional support from the endowed Bowman Professorship in Medical Science that he holds at the University of Arizona. He holds or has held research grants from the US National Institutes of Health. He holds US patent number 11119103 (serological assays for SARS-CoV-2). TG and MS were funded by the UK National Institute for Health and Care Research (LOCOMOTION study), and JŽN by the US National Institutes of Health.
The funders had no role in any aspect of the writing of this Review. The authors were not precluded from accessing any data in the study (which, being a Review, came from publicly available published papers), and they accept responsibility to submit this Review for publication.
Acknowledgments
We would like to thank Christina Pagel (Clinical Operational Research Unit, University College London) for producing figure 1 from Office of National Statistics data.
Supplementary Material (1)
PDF (255.18 KB)Supplementary appendix